Transitions between wakefulness and sleep are controlled and regulated by the brain, which also plays a key role in directing quantity and depth of sleep. However, sleep is also strongly influenced by external factors, such as light and caffeine.
Features in this section explore the basics of sleep regulation: the structures of the brain that control wakefulness and sleep, the systems that interact to enable us to stay awake and asleep for many hours at a time, and the external factors that can influence both.
Under the Brain's Control
The Takeaway
- Staying awake and alert or sleeping restfully when we choose to depends largely on the function of a few small areas of the brain.
- When the alerting areas of the brain are most active, they inhibit activity in other areas of the brain responsible for promoting sleep. This inhibition of sleep results in stable wakefulness.
- Similarly, when the sleep-promoting areas of the brain are most active, they inhibit activity in areas of the brain responsible for promoting wakefulness. This inhibition of wakefulness results in stable sleep.
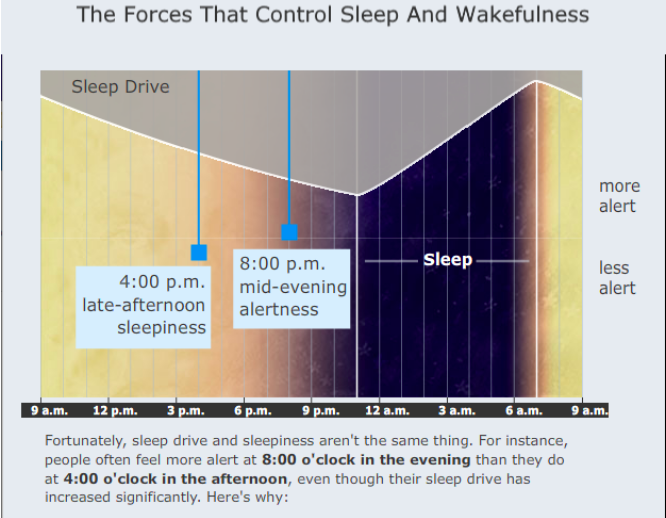
- We normally change from one stable state to the other due to internal factors, such as increasing drive to sleep that builds up during wakefulness, and changing influences from our internal biological clock.
- A number of other factors can influence the stability of this system and may cause us to fall asleep or wake up at inopportune times.
The transition is nearly instantaneous—you're awake one moment, asleep the next. This alteration of consciousness involves a swift but complex interaction between various parts of the brain. Meet the sleep switch and learn about its function.
Stable Wakefulness and Stable Sleep
In every 24-hour period, it is common for people to be continuously awake for about 16 hours and then almost continuously asleep for approximately 8 hours. A small number of brain cells are responsible for keeping us awake or asleep—some cells promote wakefulness and others promote sleep. The neurons that promote wakefulness inhibit those that promote sleep, and vice versa. This interaction normally leads to either a relatively stable period of wakefulness or a relatively stable period of sleep.
Dr. Thomas Scammell discusses how structures and chemicals in the brain are responsible for producing both wakefulness and sleep.
Although the brain's control of sleep and wakefulness is not entirely understood, scientists have pinpointed many areas of the brain involved in regulating these processes and have learned a great deal about how these areas function. For example, we now know that several areas in the brainstem and hypothalamus promote wakefulness by sending arousal signals to the cerebral cortex, the brain’s largest region. These signals come in the form of chemicals called neurotransmitters. When neurons in the arousal areas are active, the cortex remains activated and we stay awake.
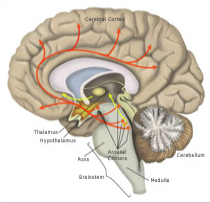
One area of the brain that promotes arousal is the tuberomammillary nucleus (TMN). Here, neurons release histamine as one of their neurotransmitters. Interestingly, many "anti-histamine" medicines block this arousing signal and cause sleepiness. Other neurons produce a neurotransmitter called orexin (also known as hypocretin), which directly stimulates the arousal centers as well as the cerebral cortex itself.
Another area of the hypothalamus is responsible for shutting down the brain’s arousal signals and causing the transition to sleep. Neurons in a part of the hypothalamus called the ventrolateral preoptic nucleus (VLPO) connect directly to the many arousal-promoting centers. Rather than stimulating activity in these areas, signals from VLPO neurons inhibit their activity. By shutting down the arousal centers, the VLPO promotes sleep.
A "Flip-Flop Switch" Between Sleep and Wakefulness
The ability to remain in a stable period of sleep or wakefulness is a result of what scientists call "mutual inhibition" between the wake-promoting neurons and the sleep-promoting neurons. So, for example, the areas of the brain that maintain wakefulness by activating the cortex also inhibit VLPO neurons. Conversely, when VLPO neurons fire rapidly and induce sleep, they also inhibit activity in the arousal centers such as the TMN.
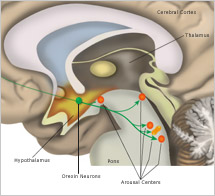
Transitions between these stable states of wakefulness and sleep occur relatively quickly, often in just seconds. Some researchers have compared the neurological mechanism that controls these rapid transitions to the "flip-flop switch" in an electrical circuit. In the brain, the mechanism that maintains stability through mutual inhibition is triggered by changes in factors such as the body's drive for sleep or the circadian alerting signal. When one of these forces becomes strong enough, it drives the transition to the opposite state. The same "flip-flop switch" analogy also describes the brain mechanisms involved in switching between rapid eye movement (REM) sleep and non-rapid eye movement (NREM) sleep. However, different neurotransmitters and different groups of neurons in the brainstem are involved in the transitions between REM and NREM sleep.
Factors That Influence These Transitions
People generally require several minutes to calm down and relax enough to fall asleep, and the deepest stages of sleep typically occur 20 or more minutes after sleep onset. However, sleep onset and associated loss of consciousness can occur in an instant. This is particularly obvious in very tired people who can fall asleep at inconvenient and sometimes dangerous times, such as when driving a car. Similarly, waking up from sleep can occur very quickly, for example in response to an alarm clock, although it typically takes people much longer to become fully alert after awakening.
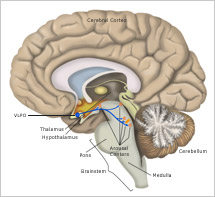
There are many internal factors (such as homeostatic sleep drive and circadian rhythms) and environmental factors (such as noise) that influence the likelihood of falling asleep or waking up. For example, a powerful sleep drive builds up with prolonged wakefulness and shifts the balance toward sleep. How this occurs is not precisely known, but adenosine is one of the chemicals thought to accumulate during prolonged wakefulness. When it does, it serves to induce sleep by inhibiting wake-promoting neurons. Interestingly, caffeine inhibits the actions of adenosine and therefore helps maintain wakefulness.
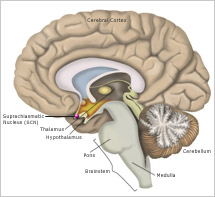
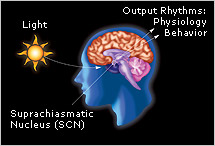
The timing of transitions between sleep and wakefulness are also tied closely to the body’s internal biological clock located in the suprachiasmatic nucleus (SCN). This tiny structure—made up of approximately 50,000 brain cells—receives light signals directly from the eye, through the optic nerve. Light resets the clock to correspond to the day-night cycle. In turn, the clock regulates the timing of dozens of different internal functions, including temperature, hormone release, and sleep and wakefulness. The SCN promotes wakefulness by producing a powerful alerting signal that offsets sleep drive. The SCN promotes sleep by turning off the alerting signal. In addition, the SCN actively maintains sleep throughout the night even after sleep drive has dissipated in the second half of the night.
References
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001; 24:726-31.
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. 2005; Nature. 437:1257–1263.
- Fuller PM, Saper CB, Lu J. The pontine REM switch: past and present. J Physiol. 2007: 584(Pt 3):735–41.