Weiss Laboratory
Department of Medicine, Beth Israel Deaconess Medical Center
Director: J. Woodrow Weiss, MD
Overview
The Weiss Laboratory has a long-standing interest in the respiratory, hemodynamic and metabolic consequences of obstructive sleep apnea. Members of the group perform basic and clinical studies to understand better how sleep disordered breathing contributes to conditions such as systemic and pulmonary hypertension, glucose intolerance and altered ventilatory control. Clinical studies are performed on patients recruited from the Sleep Disorders Center at Beth Israel Deaconess Medical Center. Additional studies are performed on human volunteers experiencing experimental sleep disruption/ sleep fragmentation or nocturnal cyclic hypoxia. Animal experiments are performed using a rodent model of cyclic intermittent hypoxia. All projects are designed to better understand how sleep apnea contributes to morbidity and mortality in affected individuals. Major collaborations exist with Dr. Andrew Taylor at Spaulding Rehabilitation Hospital in Boston and with Dr. Renaud Tamisier in Grenoble FR.
Clinical and Translational Projects
Geoffrey Gilmartin has led a series of studies over the last several years to understand how nocturnal intermittent hypoxia might contribute to sustained sympathoexcitation in sleep apnea patients. This heightened activity of the sympathetic nervous system is believed to be instrumental in the development of systemic diurnal hypertension in these individuals. Sympathetic activity is regulated by feedback from peripheral receptors (chemoreceptors and baroreceptors) and by central nervous system processing. Using a variety of models of intermittent hypoxia the group has established that exposure to intermittent hypoxia alters sympathoregulation by influencing both chemoreceptor activity and by altering baroreflex sensitivity. Recently, the group has developed a unique model of cyclic intermittent hypoxia for use in normal volunteers. This model involves having healthy subjects sleep in an altitude tent while receiving pulses of oxygen to allow oxygen saturation to oscillate overnight. Recent publications have described this model and have demonstrated that normal volunteers exposed in this manor develop an increase in waking blood pressure and increased peripheral muscle sympathetic activity. Additional studies have explored the effect of cyclic hypoxia on sleep quality and on cognitive performance.
Melanie Pogach, who recently joined the laboratory, has begun to use this model of cyclic hypoxia to explore glucose control. Patients with sleep apnea are at increased risk of developing diabetes but the mechanism of the glucose intolerance is not understood. Dr. Pogach has established that intermittent hypoxia actually improves glucose regulation after 2 weeks. She has now extended these studies to models of sleep restriction and sleep fragmentation in an attempt to explore other possible mediators of insulin resistance in sleep apnea patients.

Altitude tent to expose normal volunteers to cyclic hypoxia during sleep
Basic Studies
Patients with sleep apnea have enhanced sensitivity to hypoxia, mediated through changes in the peripheral chemoreceptor, the primary sensor of hypoxia in the body. Using a rodent model of cyclic hypoxia, the group, including Yuzhen Liu and EnSheng Ji, have begun to explore the molecular changes in the carotid body that occur after cyclic hypoxia and that lead to augmented sensitivity of the peripheral chemoreceptor. Their work has established that expression of several neuromodulators in the carotid body is altered after cyclic hypoxia. Among these neuromodulators are endothelin, angiotensin, and nitric oxide. Alterations in these neuromodulators do not appear to affect the basic oxygen sensing system but rather enhance the activity of the nerve cells that innervate the carotid body by altering the firing rate of the glomus (type 1) cells that seem to be the oxygen sensing cells. Current studies are focused on how endothelin regulates carotid chemosensitivity

Exposure chambers for rodent model of cyclic intermittent hypoxia
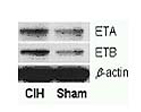
Western Blot showing an increase in expression of receptors of endothelin in carotid body after exposure to cyclic intermittent hypoxia (CIH) or a room air (Sham) exposure.
In addition to altered peripheral chemosensitivity, central sympathetic processing is altered by cyclic hypoxic exposure. Xianghong Ji and Yuzhen Liu have begun to examine how this central dysregulation occurs. Interestingly, expression of the same neuromodulators that contribute to peripheral chemosensitivity is also altered in central nervous system sites of sympathoregulation such as the paraventricular nucleus, the subfornical organ and the rostral ventral lateral medulla. Current studies are focusing on endothelin expression in the central nervous system sites and how endothelin alters sympathetic control in the neuronal systems.

Response of a Sham-exposed (A) or CIH-exposed rat (B) to central injection of endothelin showing an exaggerated response in the CIH-exposed animal (arterial pressure – red; renal sympathetic nerve activity – green and purple)
Faculty
Geoffrey S. Gilmartin, MD
J. Woodrow Weiss, MD
Affiliated Faculty
Melanie Pogach, M.D.
Yuzhen Liu, PhD
Xinghong Li, PhD.
Jingli Tong, trainee
Bethany Kulczewski, trainee
Mekkin Lynch, trainee
Renaud Tamisier, MD, collaborator (Grenoble)
Ensheng Ji, PhD, collaborator (Hebei)
Yuming Wu, PhD, collaborator (Hebei)
Trainees
Sriram Krishnamachari, PhD
Administrative Contact Name
J. Woodrow Weiss, MD
gshaidan@bidmc.harvard.edu
330 Brookline Avenue, Medicine – GZ405
Boston, MA 02215