Scammell Lab
Department of Neurology, Beth Israel Deaconess Medical Center
Director: Thomas E. Scammell, MD
Research in the Scammell Lab focuses on the neurobiology of sleep and the neural basis of narcolepsy. Narcolepsy is caused by an extensive and selective loss of the hypothalamic neurons that produce the orexin neuropeptides(also known as hypocretins). 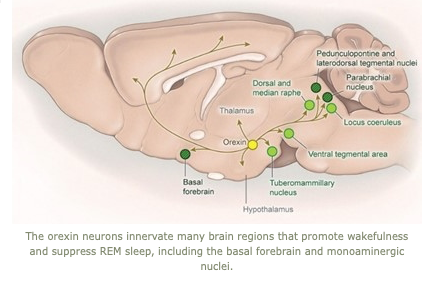 This cell loss generally occurs in the teens or young adulthood and results in lifelong sleepiness and cataplexy, brief episodes of muscle weakness that are similar to the paralysis that occurs during REM sleep. Much of our current work focuses on mouse models of narcolepsy because mice lacking orexins also have sleepiness and frequent episodes of cataplexy. We hypothesize that orexins normally stabilize the activity of wake-promoting brain regions, but absence of orexins produces behavioral state instability, with rapid transitions from wakefulness into sleep, and intrusions into wakefulness of REM sleep elements such as cataplexy or hallucinations.
This cell loss generally occurs in the teens or young adulthood and results in lifelong sleepiness and cataplexy, brief episodes of muscle weakness that are similar to the paralysis that occurs during REM sleep. Much of our current work focuses on mouse models of narcolepsy because mice lacking orexins also have sleepiness and frequent episodes of cataplexy. We hypothesize that orexins normally stabilize the activity of wake-promoting brain regions, but absence of orexins produces behavioral state instability, with rapid transitions from wakefulness into sleep, and intrusions into wakefulness of REM sleep elements such as cataplexy or hallucinations.
Our major goals are to identify the neural mechanisms through which the orexin system controls sleep and wakefulness and to determine how loss of the orexin peptides results in sleepiness and cataplexy. We are pursuing these questions in several ongoing studies:
1. Cataplexy is generally triggered by strong, positive emotions such as laughing at a great joke. The amygdala is a key site through which emotions trigger motor responses, and we are working to identify just which neurons in the amygdala mediate cataplexy. 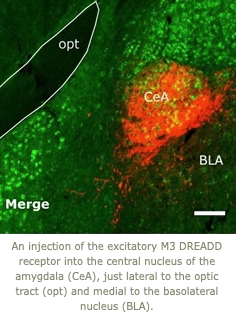 Using engineered DREADD receptors, we found that activation of GABAergic neurons in the central nucleus of the amygdala roughly doubles the amount of cataplexy whereas inhibition of these cells reduces cataplexy. We are now focused on identifying just which GABAergic neurons mediate this response to develop more targeted therapies for cataplexy.
Using engineered DREADD receptors, we found that activation of GABAergic neurons in the central nucleus of the amygdala roughly doubles the amount of cataplexy whereas inhibition of these cells reduces cataplexy. We are now focused on identifying just which GABAergic neurons mediate this response to develop more targeted therapies for cataplexy.
2. The pedunculopontine tegmental (PPT) nucleus has long been implicated in the regulation of cortical activity and behavioral states, including rapid eye-movement (REM) sleep. 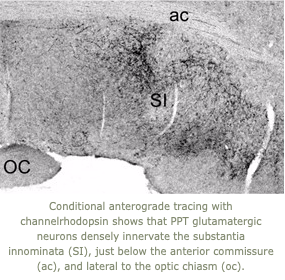 Though these effects have been linked with the activity of cholinergic PPT neurons, the PPT also includes intermingled glutamatergic and GABAergic cell populations, and the precise roles of cholinergic, glutamatergic, and GABAergic PPT cell groups in regulating cortical activity and behavioral state remain unknown. Using a chemogenetic approach in three Cre-driver mouse lines, we found that selective activation of glutamatergic PPT neurons induced prolonged cortical activation and behavioral wakefulness, whereas inhibition reduced wakefulness and increased non-REM (NREM) sleep. Activation of cholinergic PPT neurons suppressed lower-frequency electroencephalogram rhythms during NREM sleep. Last, activation of GABAergic PPT neurons slightly reduced REM sleep. We are now using optogenetics to determine the key pathways through which the glutamatergic PPT neurons promote wakefulness.
Though these effects have been linked with the activity of cholinergic PPT neurons, the PPT also includes intermingled glutamatergic and GABAergic cell populations, and the precise roles of cholinergic, glutamatergic, and GABAergic PPT cell groups in regulating cortical activity and behavioral state remain unknown. Using a chemogenetic approach in three Cre-driver mouse lines, we found that selective activation of glutamatergic PPT neurons induced prolonged cortical activation and behavioral wakefulness, whereas inhibition reduced wakefulness and increased non-REM (NREM) sleep. Activation of cholinergic PPT neurons suppressed lower-frequency electroencephalogram rhythms during NREM sleep. Last, activation of GABAergic PPT neurons slightly reduced REM sleep. We are now using optogenetics to determine the key pathways through which the glutamatergic PPT neurons promote wakefulness.
3. In collaboration with Dr. Elda Arrigoni’s lab, we are examining the electrophysiologic effects of orexin and dynorphin peptides on neurons of the basal forebrain and other regions. These studies use patch clamp recordings and channelrhodopsins to identify the precise mechanisms through which these peptides influence their targets.
4. Researchers have little understanding of just when the orexin neuropeptides are released and for how long they increase the activity of neurons bearing the orexin receptors. Defining the kinetics of orexin signaling is fundamental for understanding normal neurobiology, especially in relation to variations in arousal and sleep/wake regulation. We are using engineered cells that fluoresce when exposed to orexins to measure orexin tone in relation to changes in behavioral state, reward, and other behaviors.
5. In studies of human brains, we have found that loss of the orexin neurons in narcolepsy is also accompanied by a large increase in the number of neurons producing histamine. 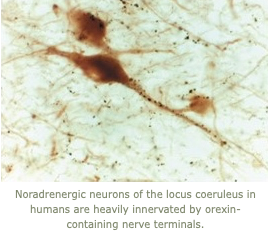 This may be a compensatory response that helps produce wakefulness after loss of the orexin neurons. In related work, we are also examining whether loss of the orexin neurons and other wake-promoting systems contributes to the sleepiness often seen after traumatic brain injury.
This may be a compensatory response that helps produce wakefulness after loss of the orexin neurons. In related work, we are also examining whether loss of the orexin neurons and other wake-promoting systems contributes to the sleepiness often seen after traumatic brain injury.
6. In collaboration with Dr. Clifford Woolf’s lab, we are also examining the interactions of sleep and pain. We have found that reductions in sleep increase behavioral responses to painful stimuli and these responses are normalized with wake-promoting medications. In addition, we have found that mice with neuropathic pain have fragmented sleep, which may be a useful biomarker of spontaneous pain.
Our lab uses a variety of anatomic, physiologic, andmolecular techniques. We frequently study sleep/wake behavior in mice using detailed analysis of the electroencephalogram in conjunction with optogenetics, photometry, DREADDs, recordings of muscle activity,locomotion, behavior, and body temperature. 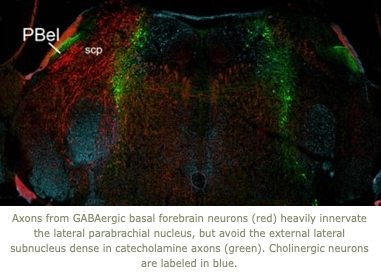 We have also developed new mathematical techniques for analysis of the transitions between behavioral states and examinationof intermediate states. We trace neural pathways using noveland conventional anterograde and retrograde tracers, and we perform immunostaining and in situ hybridization histochemistryto map the distribution of neurotransmitters, receptors, and other molecules. We also use a variety of molecular techniques to design and produce novel recombinant mice.
We have also developed new mathematical techniques for analysis of the transitions between behavioral states and examinationof intermediate states. We trace neural pathways using noveland conventional anterograde and retrograde tracers, and we perform immunostaining and in situ hybridization histochemistryto map the distribution of neurotransmitters, receptors, and other molecules. We also use a variety of molecular techniques to design and produce novel recombinant mice.
Through these approaches, we hope to gain a detailed understanding of orexin neurobiology that will result in highly effective therapies for patients with narcolepsy and enhance our knowledge of sleep.
Lab Members

Alumni/Collaborators

Lab Publications
Kroeger D, Absi G, Gagliardi C, Bandaru SS, Madara JC, Ferrari LL, Arrigoni E, Münzberg H, Scammell TE, Saper CB, Vetrivelan R. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat Commun, 2018; 9:4129.
Agostinelli, LJ, Geerling, JC, Scammell, TE. Basal forebrain subcortical projections. Brain Structure and Function, 2019;224:1097-1117.
McAlpine, CS, Kiss, M, Rattik, S, He, S, Anzai, A, Chan, CT, Mindur, JE, Valet, C, Kahles, F, Fenn, AM, Gregory, A, Halle, L, Iwamoto, Y, Hoyer, F, Binder, B, Libby, P, Scammell, TE, Nahrendorf, M, and Swirski, FK. Sleep modulates hematopoiesis and protects against atherosclerosis. Nature, 2019; 566:383-387.
Venner, A, Mochizuki, T, de Luca, R, Anaclet, C, Scammell, TE, Saper, CB, Arrigoni, E, Fuller, P. Reassessing the role of histamine- and VGAT- co-expressing tuberomammillary neurons in arousal control. Journal of Neuroscience, 2019; 39:8929-8939.
Gisabella, B, Scammell, T, Bandaru, SS, Saper, CB. Regulation of hippocampal dendritic spines following sleep deprivation. Journal of Comparative Neurology, 2020;528:380–388.
Cogswell, A, Maski, K, Scammell, T, Tucker, D, Orban, Z, Koralnik, IJ. Children with narcolepsy type 1 have increased T cell responses to orexin. Annals of Clinical and Translational Neurology, 2019; 6:2566-2572.
Lombardi, F, Gomez-Extremera, M, Bernaola-Galvan, P, Vetrivelan, R, Saper, CB, Scammell, TE, and Ivanov, PC. Critical dynamics and coupling in bursts of cortical rhythms indicate non-homeostatic mechanism for sleep-stage transitions and dual role of VLPO neurons in both sleep and wake. Journal of Neuroscience, 2020; 40:171-190.
Mahoney, C, Mochizuki, T, Scammell, TE. Dual orexin receptor antagonists increase sleep and cataplexy in wild type mice. Sleep, in press.
Maski, K, Pizza, F, Liu, S, Steinhart, E, Little, E, Colclasure, A, Diniz Behn, C, Vandi, S, Antelmi, E, Weller, E, Scammell, T, Plazzi, G. Defining Disrupted Nighttime Sleep and Assessing its Diagnostic Utility for Pediatric Narcolepsy Type 1. Sleep, in press.
Agostinelli, LJ, Mix, MR, Hefti, MM, Scammell, TE, Bassuk, AG. Input-output connections of LJA5 prodynorphin neurons. Journal of Comparative Neurology, in press.
Scammell, TE, Luo, G, Borker, O, Sullivan, L, Biddle, K, Mignot, E. Treatment of narcolepsy with natalizumab. Sleep, in press.
Insight Into Reduction of Wakefulness by Suvorexant in Patients With Insomnia: Analysis of Wake Bouts. Svetnik V, Snyder ES, Tao P, Scammell TE, Roth T, Lines C, Herring WJ. Sleep. 2018 Jan 1;41(1). PMID: 29112763
Dysregulation of Sleep Behavioral States in Narcolepsy. Schoch SF, Werth E, Poryazova R, Scammell TE, Baumann CR, Imbach LL. Sleep. 2017 Dec 1;40(12). PMID: 29029348
Decreased alertness due to sleep loss increases pain sensitivity in mice. Alexandre C, Latremoliere A, Ferreira A, Miracca G, Yamamoto M, Scammell TE, Woolf CJ. Nat Med. 2017 Jun;23(6):768-774. PMID: 28481358
Cataplexy and Its Mimics: Clinical Recognition and Management. Pillen S, Pizza F, Dhondt K, Scammell TE, Overeem S. Curr Treat Options Neurol. 2017 Jun;19(6):23. PMID: 28478511
GABAergic Neurons of the Central Amygdala Promote Cataplexy. Mahoney CE, Agostinelli LJ, Brooks JN, Lowell BB, Scammell TE. J Neurosci. 2017 Apr 12;37(15):3995-4006. PMID: 28235898
Neural Circuitry of Wakefulness and Sleep. Scammell TE, Arrigoni E, Lipton JO. Neuron. 2017 Feb 22;93(4):747-765. PMID: 28231463
Complementary roles of gasotransmitters CO and H2S in sleep apnea. Peng YJ, Zhang X, Gridina A, Chupikova I, McCormick DL, Thomas RJ, Scammell TE, Kim G, Vasavda C, Nanduri J, Kumar GK, Semenza GL, Snyder SH, Prabhakar NR. Proc Natl Acad Sci U S A. 2017 Feb 7;114(6):1413-1418. PMID: 28115703
Cholinergic, Glutamatergic, and GABAergic Neurons of the Pedunculopontine Tegmental Nucleus Have Distinct Effects on Sleep/Wake Behavior in Mice. Kroeger D, Ferrari LL, Petit G, Mahoney CE, Fuller PM, Arrigoni E, Scammell TE. J Neurosci. 2017 Feb 1;37(5):1352-1366. PMID: 28039375
Descending projections from the basal forebrain to the orexin neurons in mice. Agostinelli LJ, Ferrari LL, Mahoney CE, Mochizuki T, Lowell BB, Arrigoni E, Scammell TE. J Comp Neurol. 2017 May 1;525(7):1668-1684. PMID: 27997037
Dynamic GABAergic afferent modulation of AgRP neurons. Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, Madara JC, Campbell JN, Kroeger D, Scammell TE, Tannous BA, Myers MG Jr, Andermann ML, Krashes MJ, Lowell BB. Nat Neurosci. 2016 Dec;19(12):1628-1635. PMID: 27643429
Damage to Arousal-Promoting Brainstem Neurons with Traumatic Brain Injury. Valko PO, Gavrilov YV, Yamamoto M, Noaín D, Reddy H, Haybaeck J, Weis S, Baumann CR, Scammell TE. Sleep. 2016 Jun 1;39(6):1249-52. PMID: 27091531
Disrupted Sleep in Narcolepsy: Exploring the Integrity of Galanin Neurons in the Ventrolateral Preoptic Area. Gavrilov YV, Ellison BA, Yamamoto M, Reddy H, Haybaeck J, Mignot E, Baumann CR, Scammell TE, Valko PO. Sleep. 2016 May 1;39(5):1059-62. PMID: 26951397
Narcolepsy. Scammell TE. N Engl J Med. 2015 Dec 31;373(27):2654-62. PMID: 26716917
Systems Genomics Identifies a Key Role for Hypocretin/Orexin Receptor-2 in Human Heart Failure. Perez MV, Pavlovic A, Shang C, Wheeler MT, Miller CL, Liu J, Dewey FE, Pan S, Thanaporn PK, Absher D, Brandimarto J, Salisbury H, Chan K, Mukherjee R, Konadhode RP, Myers RM, Sedehi D, Scammell TE, Quertermous T, Cappola T, Ashley EA. J Am Coll Cardiol. 2015 Dec 8;66(22):2522-33. PMID: 26653627
Dynorphin inhibits basal forebrain cholinergic neurons by pre- and postsynaptic mechanisms. Ferrari LL, Agostinelli LJ, Krashes MJ, Lowell BB, Scammell TE, Arrigoni E. J Physiol. 2016 Feb 15;594(4):1069-85. PMID: 26613645
Genetic identity of thermosensory relay neurons in the lateral parabrachial nucleus. Geerling JC, Kim M, Mahoney CE, Abbott SB, Agostinelli LJ, Garfield AS, Krashes MJ, Lowell BB, Scammell TE. Am J Physiol Regul Integr Comp Physiol. 2016 Jan 1;310(1):R41-54. PMID: 26491097
Suppression of Locomotor Activity in Female C57Bl/6J Mice Treated with Interleukin-1β: Investigating a Method for the Study of Fatigue in Laboratory Animals. Bonsall DR, Kim H, Tocci C, Ndiaye A, Petronzio A, McKay-Corkum G, Molyneux PC, Scammell TE, Harrington ME. PLoS One. 2015 Oct 15;10(10):e0140678. PMID: 26469939
Progressive Loss of the Orexin Neurons Reveals Dual Effects on Wakefulness. Branch AF, Navidi W, Tabuchi S, Terao A, Yamanaka A, Scammell TE, Diniz Behn C. Sleep. 2016 Feb 1;39(2):369-77. PMID: 26446125
Bradysomnia in Parkinson's disease. Imbach LL, Sommerauer M, Poryazova R, Werth E, Valko PO, Scammell TE, Baumann CR. Clin Neurophysiol. 2016 Feb;127(2):1403-1409. PMID: 26419612
Overview of sleep: the neurologic processes of the sleep-wake cycle. Scammell TE. J Clin Psychiatry. 2015 May;76(5):e13. PMID: 26035194
The roles of prostaglandin E2 and D2 in lipopolysaccharide-mediated changes in sleep. Oishi Y, Yoshida K, Scammell TE, Urade Y, Lazarus M, Saper CB. Brain Behav Immun. 2015 Jul;47:172-7. PMID: 25532785
Damage to histaminergic tuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Valko PO, Gavrilov YV, Yamamoto M, Finn K, Reddy H, Haybaeck J, Weis S, Scammell TE, Baumann CR. Ann Neurol. 2015 Jan;77(1):177-82. PMID: 25363332
Usefulness of a Nocturnal SOREMP for Diagnosing Narcolepsy with Cataplexy in a Pediatric Population. Reiter J, Katz E, Scammell TE, Maski K. Sleep. 2015 Jun 1;38(6):859-65. PMID: 25325489
SCOPRISM: a new algorithm for automatic sleep scoring in mice. Bastianini S, Berteotti C, Gabrielli A, Del Vecchio F, Amici R, Alexandre C, Scammell TE, Gazea M, Kimura M, Lo Martire V, Silvani A, Zoccoli G. J Neurosci Methods. 2014 Sep 30;235:277-84. PMID: 25092499
Challenges in diagnosing narcolepsy without cataplexy: a consensus statement. Baumann CR, Mignot E, Lammers GJ, Overeem S, Arnulf I, Rye D, Dauvilliers Y, Honda M, Owens JA, Plazzi G, Scammell TE. Sleep. 2014 Jun 1;37(6):1035-42. PMID: 24882898
Optogenetic-mediated release of histamine reveals distal and autoregulatory mechanisms for controlling arousal. Williams RH, Chee MJ, Kroeger D, Ferrari LL, Maratos-Flier E, Scammell TE, Arrigoni E. J Neurosci. 2014 Apr 23;34(17):6023-9. PMID: 24760861
Delusional confusion of dreaming and reality in narcolepsy. Wamsley E, Donjacour CE, Scammell TE, Lammers GJ, Stickgold R. Sleep. 2014 Feb 1;37(2):419-22. PMID: 24501437
Carbachol excites sublaterodorsal nucleus neurons projecting to the spinal cord. Weng FJ, Williams RH, Hawryluk JM, Lu J, Scammell TE, Saper CB, Arrigoni E. J Physiol. 2014 Apr 1;592(7):1601-17. PMID: 24344163
Emerging therapeutics in sleep. Saper CB, Scammell TE. Ann Neurol. 2013 Sep;74(3):435-40. PMID: 24038193
Increase of histaminergic tuberomammillary neurons in narcolepsy. Valko PO, Gavrilov YV, Yamamoto M, Reddy H, Haybaeck J, Mignot E, Baumann CR, Scammell TE. Ann Neurol. 2013 Dec;74(6):794-804. PMID: 24006291
Orexin gene therapy restores the timing and maintenance of wakefulness in narcoleptic mice. Kantor S, Mochizuki T, Lops SN, Ko B, Clain E, Clark E, Yamamoto M, Scammell TE. Sleep. 2013 Aug 1;36(8):1129-38. PMID: 23904672
Role of the medial prefrontal cortex in cataplexy. Oishi Y, Williams RH, Agostinelli L, Arrigoni E, Fuller PM, Mochizuki T, Saper CB, Scammell TE. J Neurosci. 2013 Jun 5;33(23):9743-51. PMID: 23739971
Amygdala lesions reduce cataplexy in orexin knock-out mice. Burgess CR, Oishi Y, Mochizuki T, Peever JH, Scammell TE. J Neurosci. 2013 Jun 5;33(23):9734-42. PMID: 23739970
Control of arousal by the orexin neurons. Alexandre C, Andermann ML, Scammell TE. Curr Opin Neurobiol. 2013 Oct;23(5):752-9. 15. Review. PMID: 23683477
Narcolepsy as an adverse event following immunization: case definition and guidelines for data collection, analysis and presentation. Poli F, Overeem S, Lammers GJ, Plazzi G, Lecendreux M, Bassetti CL, Dauvilliers Y, Keene D, Khatami R, Li Y, Mayer G, Nohynek H, Pahud B, Paiva T, Partinen M, Scammell TE, Shimabukuro T, Sturkenboom M, van Dinther K, Wiznitzer M, Bonhoeffer J. Vaccine. 2013 Jan 30;31(6):994-1007. PMID: 23246545
Inter-hemispheric oscillations in human sleep. Imbach LL, Werth E, Kallweit U, Sarnthein J, Scammell TE, Baumann CR. PLoS One. 2012;7(11):e48660. PMID: 23144920
Narcolepsy: neural mechanisms of sleepiness and cataplexy. Burgess CR, Scammell TE. J Neurosci. 2012 Sep 5;32(36):12305-11. PMID: 22956821
Neural circuitry engaged by prostaglandins during the sickness syndrome. Saper CB, Romanovsky AA, Scammell TE. Nat Neurosci. 2012 Jul 26;15(8):1088-95. PMID: 22837039
Sleep neurobiology from a clinical perspective. España RA, Scammell TE. Sleep. 2011 Jul 1;34(7):845-58. PMID: 21731134
Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, Nestler EJ, Elmquist JK, Lutter M. Behav Brain Res. 2011 Sep 23;222(2):289-94. PMID: 21377495