The Takeaway
- Research has revealed that narcolepsy with cataplexy is caused by a lack of orexins (hypocretins), brain chemicals that help sustain alertness and prevent REM sleep from occurring at the wrong times.
- Genetics, age, and triggering infections or inflammation play important roles in the development of narcolepsy.
- Ongoing research is shedding light on many aspects of the disorder; as more is learned about the biology of narcolepsy, the door widens for more effective treatments.
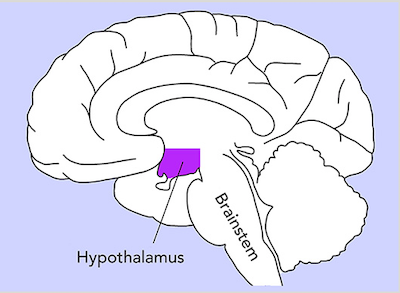
Orexins are neurotransmitters, chemicals that transmit signals from a neuron to a target neuron. Orexins are only produced by a small cluster of neurons in the hypothalamus, a brain region located roughly behind the eyes and between the ears. Of the billions of neurons in the brain, only about 100,000–200,000 produce orexins. Orexins are released from these neurons during wakefulness and bind to specific orexin receptors on target neurons, which increases the activity of these neurons.
Orexins were first discovered in 1998 when two research groups independently identified them in the brain. 1, 2 One group named them hypocretin-1 and -2, and the other group named them orexin-A and -B. (This website uses the term “orexins,” as this name is preferred by clinicians.)
Orexins and brain function
In individuals without narcolepsy and whose sleep is well regulated, orexins are released during wakefulness and increase activity in target neurons that promote wakefulness and suppress rapid-eye-movement (REM) sleep. In people who have narcolepsy with cataplexy, most of the orexin-producing neurons die off. The consequent lack of orexins results in lasting sleepiness and poor control of REM sleep. In fact, REM sleep can become so poorly regulated that the paralysis or dreaming that normally occurs only in REM sleep can mix into wakefulness, causing cataplexy and dreamlike hallucinations.
Dr. Thomas Scammell recalls the discovery of hypocretin and how it is lost in narcolepsy.
Though much has been learned about narcolepsy with cataplexy, considerably less is known about the cause of narcolepsy without cataplexy. Most likely, it is caused by less severe injury to the orexin neurons, resulting in fewer and less severe symptoms. (See Key discoveries below for more on some of the studies that have shed light on the role of orexins in narcolepsy.)
How orexin loss affects the brain
In addition to revealing the normal role of orexin neurons in the brain, research has provided many insights into how a loss of orexin signaling causes sleepiness and cataplexy.
Many researchers theorize that the sleepiness of narcolepsy is a consequence of “sleep state instability,” a condition in which the thresholds between wake and sleep are easily crossed, resulting in both fragmented wakefulness during the daytime and fragmented sleep at night.
During normal wakefulness, orexin neurons send signals that produce long-lasting increases in the activity of many other neurons essential for sustaining alertness and wakefulness. These neurons include those that produce key neurotransmitters such as norepinephrine, serotonin, and dopamine. In narcolepsy, the loss of orexins may result in reduced or inconsistent activity in these target neurons. As a consequence, people with narcolepsy can be fully alert at times, but have great difficulty sustaining this alertness for long periods of time.
Cataplexy and sleep paralysis are unusual states in which the brain circuits that produce paralysis during REM sleep become active during wakefulness. During REM sleep, most muscles are paralyzed by circuits in the lower brainstem and spinal cord. These paralysis circuits are normally blocked by norepinephrine and serotonin during wakefulness. With loss of orexins, levels of these two neurotransmitters may be lower, permitting paralysis to occur even during wakefulness. This observation provides the main rationale for treating cataplexy with antidepressants that increase brain levels of norepinephrine and serotonin.
Ongoing research is also beginning to reveal how cataplexy may be triggered by positive emotions. The amygdala and prefrontal cortex are brain regions that regulate emotional responses and connect with the paralysis pathways in the brainstem. Neurons in the amygdala and prefrontal cortex are active during cataplexy, and inactivating either of these regions markedly reduces cataplexy in mice with narcolepsy. As these triggering pathways become better understood, it may be possible to target them with new medications.
Understanding how narcolepsy develops
Over the past several years, researchers have made good progress in understanding the process that kills the orexin neurons.
Genetic factors clearly play a role. Most people with narcolepsy have inherited a gene that codes for the human leukocyte antigen (HLA) DQB1*06:02, which is important for immune function. This gene is found in 12–25% of the general population, and it increases the risk of developing narcolepsy 7- to 25-fold.3 Additional genes can increase or decrease the risk of developing narcolepsy, and, like HLA-DQB1*06:02, most of these affect the functions of the immune system. Normally, the immune system kills off bacteria and viruses. These discoveries suggest that narcolepsy is an autoimmune disease in which the immune system accidentally kills off the orexin-producing neurons.
Dr. Thomas Scammell discusses the role of a gene that appears to be important in the development of narcolepsy with cataplexy.
Researchers are now beginning to identify some of the triggers for this autoimmune attack on the orexin neurons. Just after the onset of narcolepsy, people tend to have increased levels of antibodies against streptococcus, the bacteria that causes strep throat and other infections.4 In addition, narcolepsy most commonly begins in the late spring and early summer, suggesting that an autoimmune attack on the orexin neurons may be triggered by strep or another winter infection.5
The most compelling evidence that an immune process can kill the orexin neurons comes from an event that triggered an increase in narcolepsy in Scandinavia and some other parts of Northern Europe. In the winter of 2010–2011, governments and the general public were very concerned about a severe form of influenza, known as H1N1, spreading around the globe. In many countries, most of the population received vaccination against this flu, and Finland and some other northern European countries chose to use a very potent brand of H1N1 vaccine known as Pandemrix. One to two months after receiving this brand of vaccine, dozens of children developed narcolepsy, and overall, the rate of new cases of narcolepsy in children increased 8- to 12-fold.6, 7 Importantly, all of these children who developed narcolepsy carried the gene HLA-DQB1*06:02. Only a few adults appeared to develop narcolepsy in connection with this vaccine.8 (Pandemrix, which is no longer in use anywhere, was never licensed for use in the United States.)
In the last few years, several research labs have found that adults and children with narcolepsy may have T cells, a type of immune system cell, that selectively target the orexin peptides.21-24 These T cells may directly kill the orexin neurons, or more likely, they trigger other immune cells to damage and kill the orexin neurons.
Thus, it appears that three factors are important for the development of narcolepsy:
Genes that influence the immune system, such as HLA-DQB1*06:02
A triggering factor that activates the immune system, such as infection with strep
A vulnerable age during which the immune response or some characteristics of the brain make an autoimmune attack on the orexin neurons more likely
By gaining a better understanding of this process, researchers hope to develop medications that can stop narcolepsy just as it begins and prevent further injury to the orexin neurons.
Practical implications
What are the practical implications of these discoveries? First, it is important to recognize that the hereditary aspects of narcolepsy are a relatively small concern. If a parent has narcolepsy, there is only about a 1% chance that her or his child will have the disorder. Up to 25% of the population carries the gene HLA-DQB1*06:02, but less than 1% of them will develop narcolepsy as additional factors are needed to trigger an attack on the orexin neurons. Secondly, though it seems very likely that Pandemrix triggered narcolepsy in certain children, this vaccine was unusually potent, and future vaccines most likely will be designed differently now that this risk is known. There is no compelling evidence that other vaccines cause or worsen narcolepsy, so it is fine for people with narcolepsy and their families to receive all routine vaccinations.
Key discoveries
Narcolepsy was first described by doctors in the 1870s, but only in the past 15 years has the underlying cause become clear. Some of the greatest insights about narcolepsy have come from the laboratories of Dr. Emmanuel Mignot of Stanford University and Dr. Jerome Siegel of the University of California, Los Angeles. In 2000, each group independently discovered that narcolepsy with cataplexy is caused by a loss of orexins in the brain. These and other valuable insights have revealed much about how the brain normally functions, as well as what goes wrong in narcolepsy.
Current theories about the role of orexins in narcolepsy are based on research findings that include the following:
-

Groundbreaking discoveries from Dr. Emmanuel Mignot of Stanford University (left) and Dr. Jerome Siegel of the University of California, Los Angeles, have led to many new perspectives on the nature of narcolepsy. Research in animals was the first to shed light on the connection between narcolepsy and loss of orexins. Just like people with narcolepsy, dogs, rats, and mice with disrupted orexin signaling fall asleep frequently and often have episodes of cataplexy.9, 10, 11, 12 In addition, tasty food can trigger cataplexy in dogs and mice with narcolepsy, suggesting that their cataplexy is triggered by positive emotions.13, 14
-
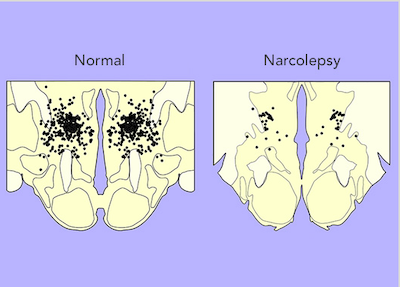
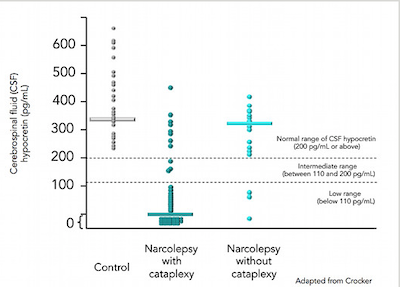
Researchers then examined the brains of people with narcolepsy with cataplexy and consistently found a 90–95% decrease in the number of orexin-producing neurons.15, 16, 17 orexin-1 can also be measured in cerebrospinal fluid, the liquid that surrounds the brain, and about 90% of people with narcolepsy with cataplexy have very low or undetectable levels of orexin-1.18 Thus, it seems likely that some process kills the orexin neurons, resulting in narcolepsy with cataplexy.
-
Thus far, the cause of narcolepsy without cataplexy is less clear. Researchers have examined the brains of only a few people with this type of narcolepsy, and these seem to have only a moderate loss of the orexin neurons.19 orexin-1 levels in spinal fluid are usually normal, although about 30% of these individuals have low or undetectable levels.18, 20 Among these people with low orexin, about one-third may develop cataplexy years later,20 suggesting that there may be some ongoing injury to the orexin neurons. Thus, narcolepsy without cataplexy may be caused by a less severe injury to the orexin neurons, but because so little is known about this form of narcolepsy, it remains possible that it has an entirely different cause.
See Developing New Treatments for the latest findings on potential new treatments for narcolepsy.
References
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 1998; 95:322–7.
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998; 92:573–85.
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51:96–100.
- Aran A, Lin L, Nevsimalova S, Plazzi G, Hong SC, Weiner K, Zeitzer J, Mignot E. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep 2009; 32:979–83.
- Han F, Lin L, Warby SC, Faraco J, Li J, Dong SX, An P, Zhao L, Wang LH, Li QY, Yan H, Gao ZC, Yuan Y, Strohl KP, Mignot E. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol 2011; 70:410–7.
- Partinen M, Saarenpaa-Heikkila O, Ilveskoski I, Hublin C, Linna M, Olsen P, Nokelainen P, Alen R, Wallden T, Espo M, Rusanen H, Olme J, Satila H, Arikka H, Kaipainen P, Julkunen I, Kirjavainen T. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS ONE 2012; 7:e33723.
- Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen SL, Hublin C, Julkunen I, Olsen P, Saarenpaa-Heikkila O, Kilpi T. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS ONE 2012; 7:e33536.
- Dauvilliers Y, Arnulf I, Lecendreux M, Monaca Charley C, Franco P, Drouot X, d'Ortho MP, Launois S, Lignot S, Bourgin P, Nogues B, Rey M, Bayard S, Scholz S, Lavault S, Tubert-Bitter P, Saussier C, Pariente A. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain 2013; 136:2486–96.
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 1999; 98:365–76.
- Beuckmann CT, Sinton CM, Williams SC, Richardson JA, Hammer RE, Sakurai T, Yanagisawa M. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci 2004; 24:4469–77.
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 1999; 98:437–51.
- Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci 2004; 24:6291–300.
- Foutz AS, Delashaw JB Jr., Guilleminault C, Dement WC. Monoaminergic mechanisms and experimental cataplexy. Ann Neurol 1981; 10:369–76.
- Oishi Y, Williams RH, Agostinelli L, Arrigoni E, Fuller PM, Mochizuki T, Saper CB, Scammell TE. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33:9743–51.
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000; 27:469–74.
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6:991–7.
- Crocker A, Espana RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, Honda M, Mignot E, Scammell TE. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology 2005; 65:1184–8.
- Mignot E, Lammers G, Ripley B, Okun M, Nevsimalova S, Overeem S, Vankova J, Black J, Harsh J, Bassetti C, Schrader H, Nishino S. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol 2002; 59:1553–62.
- Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep 2009; 32:993–8.
- Andlauer O, Moore H 4th, Hong SC, Dauvilliers Y, Kanbayashi T, Nishino S, Han F, Silber MH, Rico T, Einen M, Kornum BR, Jennum P, Knudsen S, Nevsimalova S, Poli F, Plazzi G, Mignot E. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep 2012; 35:1247–55F.
- Latorre D, et al. T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature. 2018; 562:63-8.
- Kornum BR, et al. Absence of autoreactive CD4_ T cells targeting HLA-DQA1*:02/DQB1*06:02 restricted hypocretin/orexin eipitopes in narcolepsy type 1 when detected by EliSpot. J Neuroimmunol. 2017; 309:7-11.
- Luo G, et al. Absence of anti-hypocretin receptor 2 autoantibodies in post Pandemrix narcolepsy cases. PLoS One. 2017;12:e0187305.
- Cogswell AC, et al. Children with narcolepsy type 1 have increased T-cell responses to orexins. Ann Clin Transl Neurol. 2019; 6:2566-72.
back to Narcolepsy Home